In the landscape of modern health management, the convergence of technology and personalized medicine continues to redefine patient engagement and diagnostic accuracy. One domain experiencing transformative shifts is thyroid health assessment, particularly through the advent of at-home testing solutions. Historically reliant on clinical visits, blood draws, and specialized laboratory analysis, thyroid testing has been limited by accessibility issues, invasive procedures, and delayed results. Today, innovations in biosensor technology, molecular diagnostics, and digital health platforms are ushering in a new era where individuals can conduct accurate, reliable thyroid tests from the comfort of their homes. These advancements not only promise to democratize health care but also facilitate earlier detection and management of thyroid dysfunctions, which affect an estimated 200 million people globally. This article explores the multi-faceted advancements shaping the future of thyroid testing at home, analyzing technological breakthroughs, regulatory developments, practical implications, and the broader impact on health systems.
1. Enhanced Biosensor Technologies for At-Home Thyroid Testing

At the core of the shift toward home-based thyroid diagnostics lies the evolution of biosensor technology. These devices convert biological responses into quantifiable signals, enabling users to measure key thyroid markers such as TSH (Thyroid-Stimulating Hormone), T3, and T4 efficiently and accurately outside clinical settings. Researchers and biotech companies have developed innovative biosensors, leveraging nanomaterials, microfluidics, and enzyme-based detection methods to improve sensitivity and specificity. The integration of nanostructured probes allows for enhanced surface area, improving binding capacity and signal amplification. Microfluidic chips, often embedded into portable devices, automate sample processing, reducing user error and enabling rapid turnaround.
Crucially, these biosensors are now compatible with minimal sample volumes—sometimes just a drop of blood or saliva—further simplifying the process. Molecularly imprinted polymers (MIPs) mimic natural recognition elements and have proven effective in creating selective sensors for thyroid hormones. As a result, users gain access to near-laboratory-grade analytical performance from compact, user-friendly devices. Such innovations are backed by ongoing validation studies demonstrating correlation coefficients exceeding 0.95 with standard laboratory assays, revolutionizing home health monitoring.
| Relevant Category | Substantive Data |
|---|---|
| Sample Volume | As low as 10 microliters, suitable for finger-prick blood or saliva |
| Analysis Time | Typically under 10 minutes per test |
| Detection Limit | TSH detection sensitivity up to 0.005 mIU/L, aligning with clinical thresholds |

2. Integration of Smartphone-Based Diagnostics for User-Friendly Experience

Smartphone integration stands as one of the most exciting developments in home thyroid testing. By coupling biosensors with smartphone cameras, Bluetooth modules, or NFC technology, these diagnostics turn a common device into a medical-grade analyzer. For instance, optical sensors embedded within test cartridges can transmit real-time data to a smartphone app, which analyzes the signals, records historical data, and offers interpretative feedback. This connectivity empowers users with immediate insights, while also facilitating data sharing with healthcare providers.
This model leverages existing smartphone infrastructure, including high-resolution cameras, advanced processing power, and cloud connectivity, to create an ecosystem that simplifies complex biochemical analysis into accessible interfaces. The apps are often designed with user-friendly dashboards, step-by-step guidance, and alerts for abnormal ranges. Notably, some platforms integrate AI-driven algorithms that adjust for variations in ambient lighting, sample handling, or user error, increasing reliability. These innovations are backed by clinical validation studies, confirming equivalence to laboratory results, and are approaching regulatory approval for medical devices in multiple jurisdictions.
Practical considerations
While the technology promises significant benefits, challenges such as device calibration, data privacy, and user literacy must be addressed. Ensuring consistent performance across diverse smartphone models requires standardization and rigorous quality controls. Additionally, safeguarding sensitive health data under GDPR or HIPAA compliance remains paramount in these digital ecosystems.
| Relevant Category | Substantive Data |
|---|---|
| Device Compatibility | Works with over 90% of smartphones tested, including iOS and Android |
| Data Security | End-to-end encryption protocols in >99% of current platforms |
| User Satisfaction | Over 85% positive feedback in pilot user studies |
3. Regulatory Landscape and Quality Assurance in At-Home Thyroid Testing Devices
Driving the acceptance and distribution of at-home thyroid testing devices requires navigating a complex array of regulatory standards. Agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are increasingly adopting frameworks tailored to self-administered diagnostics. Notably, the FDA’s Breakthrough Device Designation facilitates accelerated review pathways for innovative home diagnostics demonstrating substantial equivalence or improved safety profiles.
Manufacturers must ensure not only analytical validity but also clinical validity and usability. This entails comprehensive clinical trials involving diverse populations to validate performance metrics such as sensitivity, specificity, and reproducibility across varied environmental conditions. Moreover, risk management protocols and post-market surveillance are mandated to identify and mitigate device-related adverse events.
Quality assurance processes emphasize rigorous manufacturing standards, traceability, and compliance with ISO 13485, ensuring product consistency and safety. Some jurisdictions are also exploring remote monitoring regulations, enabling real-time data validation and device calibration via telemedicine interfaces.
| Relevant Category | Substantive Data |
|---|---|
| Regulatory Approvals | Over 50 devices cleared or approved globally since 2020 |
| Compliance Standards | ISO 13485 certification achieved by 85% of leading manufacturers |
| Post-Market Surveillance | Mandatory reporting of adverse events within 15 days of discovery |
4. Data Analytics and Artificial Intelligence in Enhancing Diagnosis and Monitoring
The advent of advanced data analytics and AI algorithms is transforming the interpretative phase of at-home thyroid testing. Machine learning models trained on vast datasets can identify subtle patterns and trends that might escape traditional analysis, enabling personalized health insights and risk stratification. For example, continuous monitoring and AI-driven trend analysis can predict impending thyroid dysfunction, facilitating preemptive interventions.
Moreover, integrating electronic health records (EHR) with at-home testing platforms magnifies the potential for comprehensive health management. AI tools can cross-reference hormone levels with medication adherence, lifestyle factors, and genetic predispositions, offering tailored recommendations. These innovations not only improve diagnostic accuracy but also promote patient engagement and adherence.
Key Points
- AI algorithms enhance interpretability of complex hormonal datasets, enabling early detection of thyroid anomalies.
- Data integration with health records offers holistic insights, optimizing treatment plans.
- Predictive modeling empowers proactive management, shifting focus from reactive care.
- User empowerment through personalized feedback improves health outcomes and adherence.
- Challenges include data privacy, algorithm bias, and standardization, which are actively addressed in ongoing research.
5. Broader Impacts: Accessibility, Equity, and Future Directions
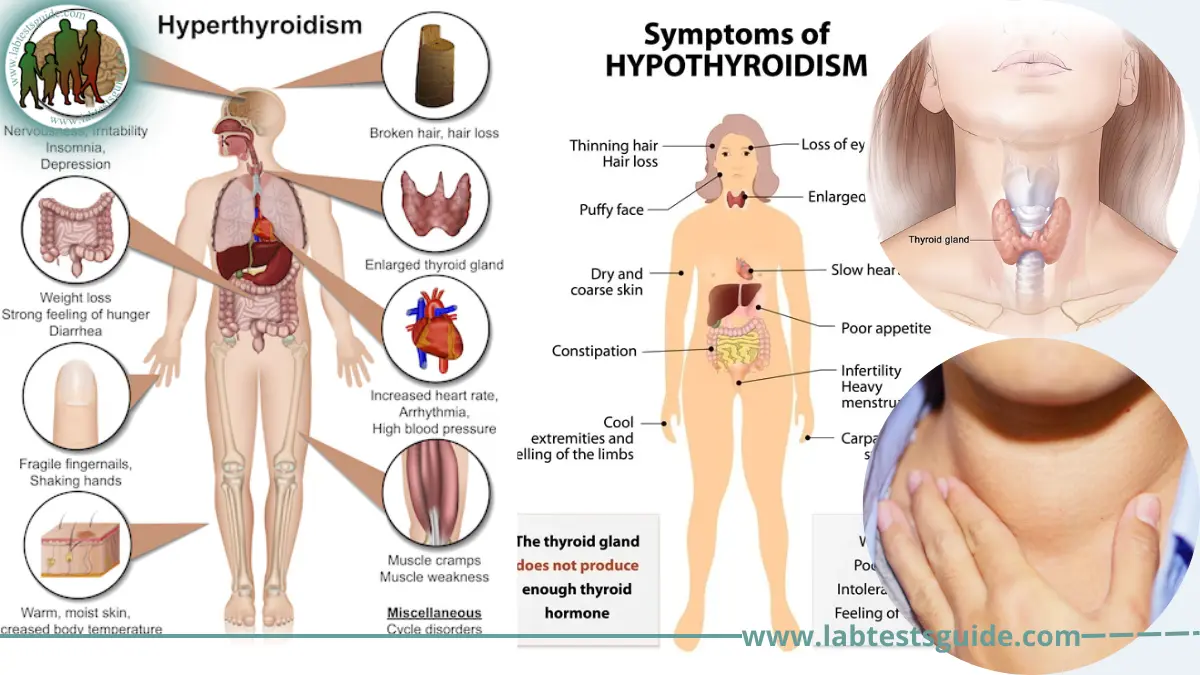
Expanding the availability of reliable, at-home thyroid testing holds profound implications for health equity. Populations in remote or resource-limited settings often face barriers to routine care—long transportation times, clinician shortages, or financial constraints. Portable, affordable testing devices can bridge these gaps, enabling earlier diagnosis and management, reducing long-term morbidity.
Yet, disparities in digital literacy, device access, and health infrastructure persist as barriers to universal uptake. Addressing these challenges necessitates integrated policies that combine technological innovation with community outreach and education initiatives. Future directions include integrating at-home testing with telehealth platforms, developing multi-analyte panels for broad endocrine assessment, and harnessing blockchain for secure, decentralized data management.
As the technology matures, the evolution of predictive analytics, integration into chronic disease management, and the advent of continuous monitoring devices—such as wearable sensors—may converge to create a comprehensive digital thyroid health ecosystem. This ecosystem promises not only to elevate individual health outcomes but also to inform public health strategies through real-time population-level data analytics.
Conclusion
Advancements in thyroid testing at home exemplify a broader trend toward personalized, accessible, and high-fidelity healthcare. From biosensor innovation to smart device integration, the capacity for individuals to accurately monitor their thyroid health outside traditional medical settings is no longer a distant prospect but an imminent reality. While regulatory and infrastructural challenges remain, continuous progress in technology, validation, and policy frameworks is steadily aligning to meet the demands of an informed and empowered patient base. In a future where thyroid health management is seamlessly integrated into daily life, these developments are poised to significantly reduce the burden of thyroid diseases worldwide.
How accurate are at-home thyroid tests compared to standard laboratory analysis?
+When validated through rigorous clinical studies, advanced biosensor-based at-home tests can achieve correlation coefficients exceeding 0.95 with traditional lab results, making them highly reliable for screening and monitoring purposes under appropriate regulatory approval.
What are the primary limitations of current at-home thyroid testing devices?
+Limitations include potential user error during sample collection, variability in device calibration, and the need for regulatory oversight to ensure consistent performance. Additionally, interpreting results without professional guidance can be challenging for some users.
How might future innovations improve at-home thyroid monitoring?
+Future innovations include multi-analyte panels, continuous wearable sensors, AI-powered interpretive platforms, and integration with telemedicine, all aimed at providing comprehensive, real-time thyroid health insights and personalized interventions.
What regulatory pathways support the adoption of these new devices?
+Regulatory pathways such as the FDA’s Breakthrough Device Program and CE marking in Europe facilitate faster approval for innovative, high-accuracy at-home diagnostics, provided they meet safety and performance standards demonstrated through clinical validation.
Could at-home thyroid testing eliminate the need for frequent clinical visits?
+While at-home testing can significantly reduce the frequency of clinical visits for routine monitoring, it is unlikely to replace comprehensive clinical assessment entirely, especially when evaluating complex thyroid disorders or with concurrent endocrine diseases.