The human body is a marvel of biological engineering, with intricate systems working harmoniously to maintain balance, coordination, and responsiveness. Among these systems, proprioception—the sense that allows us to perceive the position, movement, and force exerted by our limbs—relies on specialized sensory structures located throughout our musculoskeletal framework. One of the lesser-known yet highly significant components of this sensory network is the Golgi tendon organ (GTO). Despite its relatively small size, the Golgi tendon organ plays a pivotal role in regulating muscle tension and protecting tendons from injury, functioning as an essential feedback mechanism in motor control. Unraveling the complex operation of Golgi tendon organs involves a deep dive into neurophysiology, biochemical signaling, and biomechanical integration, making their study a prime example of multidisciplinary scientific inquiry.
Introduction to Golgi Tendon Organs: Anatomical and Functional Context

The Golgi tendon organ is a proprioceptive receptor situated at the junction where muscle fibers meet the tendons—specifically within the musculotendinous junction. Unlike muscle spindles, which primarily detect muscle stretch and length, GTOs are specialized to sense the tension exerted by muscle contractions. This distinction is vital because it provides the nervous system with critical information about the force being generated, not just the position of a limb. Anatomically, GTOs consist of a capsule enclosing a network of sensory nerve fibers intertwined with collagen fibers, creating a highly responsive yet resilient structure adapted to continual mechanical fluctuations.
Functionally, Golgi tendon organs are considered an integral component of the body’s built-in protective mechanism against excessive muscle tension. When a muscle contracts with force beyond a certain threshold, the GTOs detect this increase in tension and send afferent signals to the central nervous system. This feedback helps modulate muscle activity, thereby preventing potential tissue damage from overexertion or sudden load changes. The evolutionary significance of this function is evident across species, highlighting the GTO’s role in survival and physical resilience.
Mechanisms of Action: How Golgi Tendon Organs Operate
At the core of Golgi tendon organ activity lies the transduction process—converting mechanical tension into electrical signals that the nervous system can interpret. When muscle contraction increases, tension transmitted through the collagen fibers within the GTO capsule causes deformation of the sensory nerve endings. This deformation opens mechanosensitive ion channels—primarily sodium and potassium channels—leading to depolarization of the afferent nerve fibers. Once depolarized, these fibers generate action potentials that propagate toward the spinal cord via group Ib afferent fibers, which are characterized by their large diameter and rapid conduction velocity.
The afferent signals are transmitted to the dorsal horn of the spinal cord, where they synapse with interneurons and, ultimately, motoneurons governing the same muscle group. This feedback loop—often described as the Golgi tendon reflex—is critical for fine control over muscle force output. When tension exceeds safe limits, the reflex inhibits the alpha motoneurons, causing muscle relaxation and protecting the tissue from injury. Conversely, during normal movement, this system provides a dynamic calibration, preventing both underperformance and overexertion.
This operation exemplifies a negative feedback control system, a hallmark feature in biological regulation. Importantly, the response threshold varies among individuals and can be modulated through learning, training, or even pathological states, adding layers of complexity to how GTOs contribute to motor function.
Biochemical and Electrophysiological Signatures of GTO Activation
Activation of Golgi tendon organs involves a cascade of biochemical and electrophysiological events. Mechanical deformation of nerve endings leads to the opening of stretch-sensitive ion channels. Recent research emphasizes the role of mechanosensitive molecules, such as piezo channels, which are expressed in sensory nerve endings and respond rapidly to mechanical stimuli. The resulting influx of ions causes a local depolarization that can reach the threshold for action potential initiation.
Electrophysiologically, the firing rate of group Ib fibers correlates with the tension levels in the tendon—firing rates increase with higher tension and decrease during relaxation. Advanced neurophysiological techniques, like microneurography, have allowed researchers to record these signals in vivo, providing insight into how GTO activity aligns with force modulation during dynamic movements.
| Relevant Category | Substantive Data |
|---|---|
| Activation threshold | Typically around 20-30 N of tension in human tendons, with variation depending on muscle group and individual physiology |
| Firing rate during maximal contraction | Group Ib afferent fibers can reach firing rates of up to 250 impulses per second, indicating a highly sensitive feedback system |
| Response latency | Dependent on conduction velocity, generally around 10-25 ms from tension onset to afferent firing in humans |
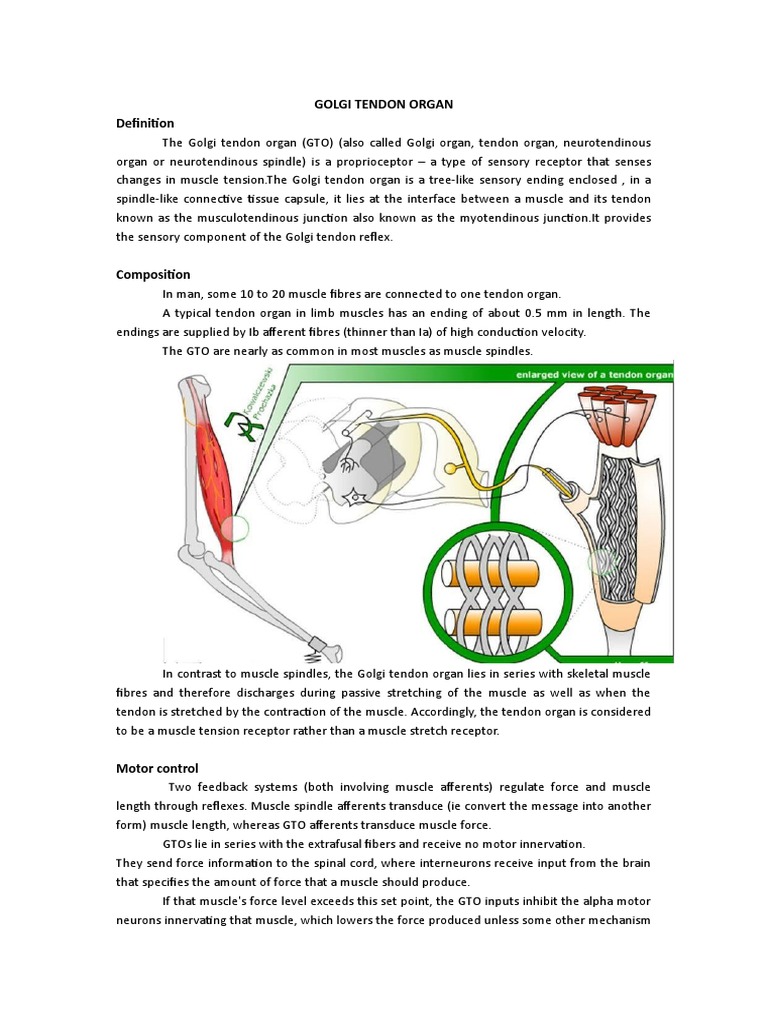
Role in Movement Regulation and Motor Learning
Golgi tendon organs are not merely passive sensors; they actively shape motor output through their reflexive responses. During voluntary movements, GTO feedback helps calibrate muscle forces, ensuring smooth and controlled actions. For example, during weightlifting, the GTO prevents excessive load on tendons and muscles by signaling tension overload, triggering relaxation in the contracting muscle. Such reflexes are integral to what physical therapists and coaches refer to as dynamic stability and safe exertion limits.
Moreover, GTO activity contributes to motor learning and adaptation. Studies involving skill acquisition demonstrate that training can modify the sensitivity threshold of GTOs, effectively increasing or decreasing their firing response to tension. This plasticity underpins the ability to adapt to new movement patterns, optimize performance, or recover from injury-related proprioceptive deficits.
In clinical contexts, GTO reflexes are leveraged in therapies like proprioceptive neuromuscular facilitation (PNF), where specific stretching and contraction techniques aim to harness GTO-mediated relaxation to enhance flexibility and restore movement patterns. Furthermore, understanding GTO function informs the design of advanced prosthetic devices and exoskeletons, where artificial sensors emulate natural proprioception for more intuitive control.
Implications for Rehabilitation and Athletic Training
In sports science and rehabilitation, deliberate modulation of GTO activity can significantly influence outcomes. Athletes train their proprioceptive systems through complex movement patterns that recalibrate GTO thresholds, thus enabling higher force outputs without risking injury. Conversely, therapeutic interventions seek to retrain or compensate for damaged GTO pathways to restore safe movement sensations.
Emerging technologies, such as wearable sensors and biofeedback systems, now aim to provide real-time data on tendon tension, assisting individuals in maintaining optimal load levels during activity. The interplay between neural feedback and biomechanical forces continues to be an expansive frontier, with ongoing research aimed at unlocking the full potential of proprioceptive modulation in health and disease.
Limitations and Opportunities for Future Research
Despite extensive understanding, several questions about Golgi tendon organs remain unresolved. For instance, the precise molecular identity of the stretch-sensitive ion channels, their regulation under various physiological and pathological states, and how central nervous system modulation impacts GTO sensitivity are areas ripe for investigation. Notably, individual variability in GTO thresholds could influence susceptibility to injury or performance capacity—a hypothesis supported by evidence showing genetic and training-related adaptations.
Furthermore, as neuroengineering and robotics advance, replicating GTO-like feedback in artificial systems holds potential to revolutionize prosthetics and exoskeletons. Integration of bio-hybrid sensors mimicking GTO function may allow machines to adapt dynamically to load changes, improving safety and performance in biomechanical interfaces.
Finally, exploring how aging affects GTO function and proprioceptive acuity is vital for developing interventions for fall prevention and mobility preservation in older populations. Overall, the convergence of neurobiology, biomechanics, and engineering promises to deepen our understanding and application of Golgi tendon organ principles.