Imagine peering into the microscopic world where atoms engage in an intricate dance to achieve stability—each step governed by precise energetic exchanges and quantum rules. This unseen universe underpins the very fabric of our daily materials, from the salts in our diet to the complex biomolecules that sustain life. Among the myriad interactions, covalent and ionic bonds represent two fundamental pathways through which atoms forge molecular structures. While they exhibit distinct behaviors and formation mechanisms, their comparative strengths, applications, and stability profiles dictate their utility across chemistry and industry. Unlocking the mechanics of whether covalent or ionic bonds are "better" isn't merely an academic debate; it provides insights that influence materials science, pharmaceuticals, nanotechnology, and beyond.
The Foundations of Bond Formation: Covalent and Ionic Bonds
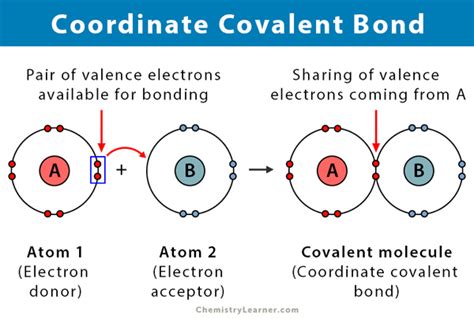
Atoms seek electron configuration stability, often following the octet rule, which states that atoms tend to prefer a full outer shell of eight electrons. The routes they take to achieve this can be broadly classified into covalent and ionic bonds, distinguished by the nature of electron sharing and transfer processes involved.
Mechanics of Covalent Bonding
A covalent bond forms when two atoms share one or more pairs of electrons. This sharing results from similar electronegativities—an atom’s tendency to attract electrons—causing a mutual stabilization as their electron clouds overlap. Typically, nonmetallic elements such as carbon, nitrogen, oxygen, and halogens form covalent bonds, creating molecules with specific directional properties. Covalent bonds involve the quantum mechanical overlap of atomic orbitals, leading to localized electron density between nuclei, which is responsible for the precise shapes of molecules, such as the tetrahedral geometry of methane or the planar structure of benzene.
Principles Behind Ionic Bonding
Ionic bonds emerge from the electrostatic attraction between oppositely charged ions generated when one atom transfers electrons to another. This process is driven by a significant difference in electronegativities, commonly observed between metals and nonmetals—metals tend to lose electrons, forming cations, whereas nonmetals gain electrons, forming anions. The classic example of ionic bonding involves sodium chloride (NaCl). Upon losing an electron, sodium becomes Na+; chlorine gains that electron, becoming Cl−. The resultant ionic lattice is held together by a balanced array of Coulombic forces, producing crystalline structures with high melting points and considerable hardness.
| Relevant Category | Substantive Data |
|---|---|
| Bond Energy | Average covalent bond energy ranges from 50 to 110 kcal/mol, while ionic bonds often exceed 150 kcal/mol depending on the lattice energy. |
| Bond Length | Covalent bonds have bond lengths typically between 1.0–2.0 Å, whereas ionic compound bond distances depend on lattice parameters but are generally larger due to electrostatic interactions. |
| Electrical Conductivity | Solutions of ionic compounds conduct electricity effectively, as mobile ions are present, whereas covalent compounds are often insulators unless they ionize or dissociate in solution. |

Energy and Stability: Which Bond Formation Outperforms?

Determining which bonding process—covalent or ionic—is “better” depends heavily on context, particularly the application’s demands for stability, reactivity, and physical properties. Covalent bonds excel in scenarios requiring directional specificity and flexibility, such as in complex organic molecules, pharmaceuticals, and polymers. Conversely, ionic bonds foster inorganic lattices with high melting points, hardness, and solubility traits crucial in industrial contexts.
Bond Strength and Thermodynamic Considerations
Bond energy, a metric quantifying the energy required to break a bond, provides a comparative lens. Covalent bonds typically range from 50–110 kcal/mol, reflecting their shared electron nature. Ionic bonds, fortified by lattice energy—the energy released when gaseous ions form an ionic solid—generally surpass covalent bonds in strength, often by a significant margin. For NaCl, lattice energy estimates hover around 787 kJ/mol, indicative of a formidable electrostatic network.
However, bond strength is only part of the story. The enthalpy of formation, solubility profiles, and kinetic stability influence practical stability for real-world uses, especially under varying environmental conditions. Covalent compounds tend toward stability in organic solvents, whereas ionic compounds often dissolve readily in polar solvents like water—a critical consideration in chemical manufacturing and biological systems.
Reactivity and Environmental Influences
Reactivity profiles further distinguish covalent from ionic bonds. Covalent molecules can undergo diverse reactions—addition, substitution, redox—often enabling complex synthesis routes. Ionic compounds might react via dissolution and subsequent ion exchange or precipitation but are less prone to covalent-type transformations unless mediated through lattice defects or surface interactions.
| Metric | Covent vs. Ionic |
|---|---|
| Melting Point | Typically lower for covalent substances (<200°C), higher for ionic salts (>700°C) |
| Solubility | Varies widely; many covalent compounds are insoluble in water, while ionic salts are highly soluble in polar solvents |
| Electrical Conductivity | Poor in solids for covalent compounds; high in aqueous solutions of ionic salts |
Application Spectrum: Contexts Favoring Covalent or Ionic Bonds
Different industry sectors and scientific disciplines leverage each bond type’s strengths. Organic chemistry, pharmaceuticals, and polymer science depend heavily on covalent interactions for molecular specificity and stability. Ionic bonding, meanwhile, underpins mineralogy, ceramics, and electrochemical devices, where high stability and ionic conductivity are desirable.
Covalent Bonds in Organic and Medicinal Chemistry
The versatility of covalent bonds enables the molecular diversity necessary in drug design, catalysis, and materials with tailored properties. Their directional nature allows precise structural control. For instance, peptide bonds in proteins exemplify strong covalent linking through amide bonds, critical for biological function.
Ionic Bonds in Materials and Electrochemistry
ionic bonds produce crystalline solids such as salts and ceramics with remarkable thermal stability and hardness. These properties are essential in manufacturing insulators, refractory materials, and electrolytes for energy storage systems. Moreover, ionic conduction in solid electrolytes is a frontier for safer, more efficient batteries, replacing liquid electrolytes that pose flammability risks.
Evaluating ‘Better’: A Context-Dependent Paradigm
Labeling one bonding type as universally superior oversimplifies the rich, domain-specific criteria that define their efficacy. Bonding strength, stability, solubility, reactivity, and application demands all influence which is deemed “better” in a given scenario. For example, covalent bonds enable the complexity needed in organic frameworks, while ionic bonds offer robustness and high thermal resistance suited for inorganic materials.
Looking Ahead: Hybrid and Emerging Bonding Paradigms
Modern material science explores hybrid bonding environments, combining covalent and ionic features for multifunctional materials. Metal-organic frameworks (MOFs) and covalent-organic frameworks (COFs) merge the stability and tunability of covalent networks with ionic interactions, unlocking new avenues in gas storage, catalysis, and sensing. Similarly, advances in computational chemistry allow engineers to tailor bond characteristics at the molecular level, optimizing stability and performance in myriad applications.
Key Points
- Understanding the bonding mechanisms is essential for designing materials with desired physical and chemical properties.
- Energy considerations reveal that ionic bonds often outperform covalent bonds in strength, yet the context determines their practical utility.
- Application-driven selection hinges on the specific environmental stability, reactivity, and physical characteristics needed.
- Hybrid bonds and advanced frameworks demonstrate the ongoing evolution of bonding paradigms, merging the best of both worlds.
- Innovative material science leverages nuanced understanding of bond types to develop next-generation technologies.
What factors influence whether covalent or ionic bonding is preferred for a material?
+Pathways such as electronegativity differences, desired physical properties (e.g., hardness, flexibility), solubility, and thermal stability guide the selection. Covalent bonds favor complex organic structures, while ionic bonds excel in inorganic, crystalline materials with high thermal resistance.
Can bonds transition from covalent to ionic or vice versa?
+While the fundamental nature of a bond is dictated by the elements involved, environmental factors like polarity, solvents, and temperature can influence bond character, pushing systems toward more covalent or ionic behavior, especially in polarizable systems or during phase transitions.
How do hybrid materials utilize both covalent and ionic bonds?
+Hybrid materials such as MOFs and COFs employ covalent bonds for structural backbone stability with ionic interactions for functionalities like ion conduction or responsive behavior, enabling applications in catalysis, sensing, and energy storage.
