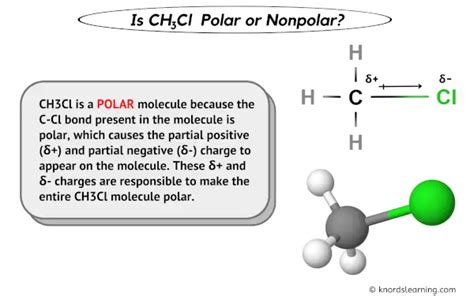
The determination of whether a molecule is polar or nonpolar is crucial in understanding its chemical and physical properties. One such molecule is CH3Cl, also known as chloromethane or methyl chloride. To classify CH3Cl as polar or nonpolar, we must first understand the concepts of polarity and the factors that influence it.
Understanding Polarity

Polarity in a molecule arises from the difference in electronegativity between atoms in a covalent bond. Electronegativity is a measure of an atom’s ability to attract electrons towards itself in a covalent bond. When there is a significant difference in electronegativity between the atoms in a bond, the bond is considered polar. This results in a partial positive charge on one side of the bond and a partial negative charge on the other, creating a dipole moment.
Electronegativity Values
The electronegativity values of the atoms in CH3Cl are crucial for determining its polarity. The electronegativity of carbon © is approximately 2.5, hydrogen (H) is about 2.2, and chlorine (Cl) is around 3.0. The significant difference in electronegativity between carbon and chlorine (0.5 units) indicates that the C-Cl bond is polar.
| Atom | Electronegativity Value |
|---|---|
| Carbon (C) | 2.5 |
| Hydrogen (H) | 2.2 |
| Chlorine (Cl) | 3.0 |

Molecular Geometry of CH3Cl
The molecular geometry of CH3Cl is tetrahedral, with the carbon atom at the center bonded to three hydrogen atoms and one chlorine atom. This geometry is asymmetrical due to the difference in the size and electronegativity of the chlorine atom compared to the hydrogen atoms. As a result, the dipoles associated with the C-H bonds do not completely cancel out the dipole of the C-Cl bond, leading to a net dipole moment for the molecule.
Dipole Moment
The dipole moment is a measure of the separation of positive and negative electrical charges within a molecule. It is expressed in Debye units (D). The dipole moment of CH3Cl is approximately 1.86 D, indicating that it has a significant permanent electric dipole moment. This value supports the classification of CH3Cl as a polar molecule.
Key Points
- The difference in electronegativity between carbon and chlorine in CH3Cl leads to a polar C-Cl bond.
- The molecular geometry of CH3Cl is tetrahedral, which is asymmetrical due to the presence of the chlorine atom.
- The molecule has a net dipole moment, classifying it as polar.
- The polarity of CH3Cl affects its physical and chemical properties, such as its boiling point and solubility.
- Understanding the polarity of molecules like CH3Cl is essential for predicting their behavior in various chemical reactions and applications.
In conclusion, CH3Cl is a polar molecule due to the significant difference in electronegativity between the carbon and chlorine atoms, leading to a polar C-Cl bond, and its asymmetrical tetrahedral molecular geometry, which results in a net dipole moment. The polarity of CH3Cl is crucial for understanding its chemical and physical properties and predicting its behavior in different scenarios.
What is the primary factor that determines the polarity of a molecule?
+The primary factor is the difference in electronegativity between the atoms in a covalent bond. A significant difference leads to a polar bond.
How does the molecular geometry of CH3Cl contribute to its polarity?
+The tetrahedral geometry of CH3Cl is asymmetrical due to the chlorine atom, resulting in a net dipole moment because the dipoles of the C-H bonds do not completely cancel out the dipole of the C-Cl bond.
What is the significance of the dipole moment in classifying a molecule as polar or nonpolar?
+A molecule with a significant dipole moment is considered polar. The dipole moment of CH3Cl is approximately 1.86 D, indicating it is polar.