In the intricate world of chemical nomenclature, mastering acid naming rules stands as a foundational skill critical for students, educators, and professionals alike. The process requires a nuanced understanding of systematic conventions, including the identification of acid types—binary and oxyacids—and the application of specific suffixes and prefixes. As scientific communication advances, the ability to accurately name acids not only enhances clarity but also fosters effective collaboration across disciplines. In this detailed exploration, we will compare and contrast the fundamental principles governing acid nomenclature, delve into the historical evolution of these rules, and analyze their practical applications within modern chemical practices.
Understanding Basic Acid Naming Conventions: Binary vs. Oxyacids

The classification of acids primarily falls into two categories: binary acids and oxyacids. Each follows distinct naming principles, shaped by their chemical structure and constituent elements. Binary acids consist of hydrogen combined with a nonmetal, such as fluorine, chlorine, or sulfur. Oxyacids, by contrast, incorporate hydrogen, oxygen, and a central nonmetal element, often involving complex polyatomic ions.
Binary Acid Naming: Simplicity in Structure, Complexity in Procedure
Binary acids are straightforward in their composition but require precise nomenclature rules to avoid ambiguity. The nomenclature convention involves prefixing the word “hydro-” to the root of the nonmetal element, followed by the suffix “-ic,” and then the word “acid.” For instance, hydrogen fluoride becomes hydrofluoric acid, hydrogen chloride transforms into hydrochloric acid, and hydrogen sulfide is named hydrosulfuric acid. This structure emphasizes the presence of hydrogen directly bonded to a nonmetal, reflecting the pure binary nature of these acids.
| Relevant Category | Substantive Data |
|---|---|
| Standard Naming Pattern | Hydro- + nonmetal + -ic + acid |
| Example: HF | Hydrofluoric acid |
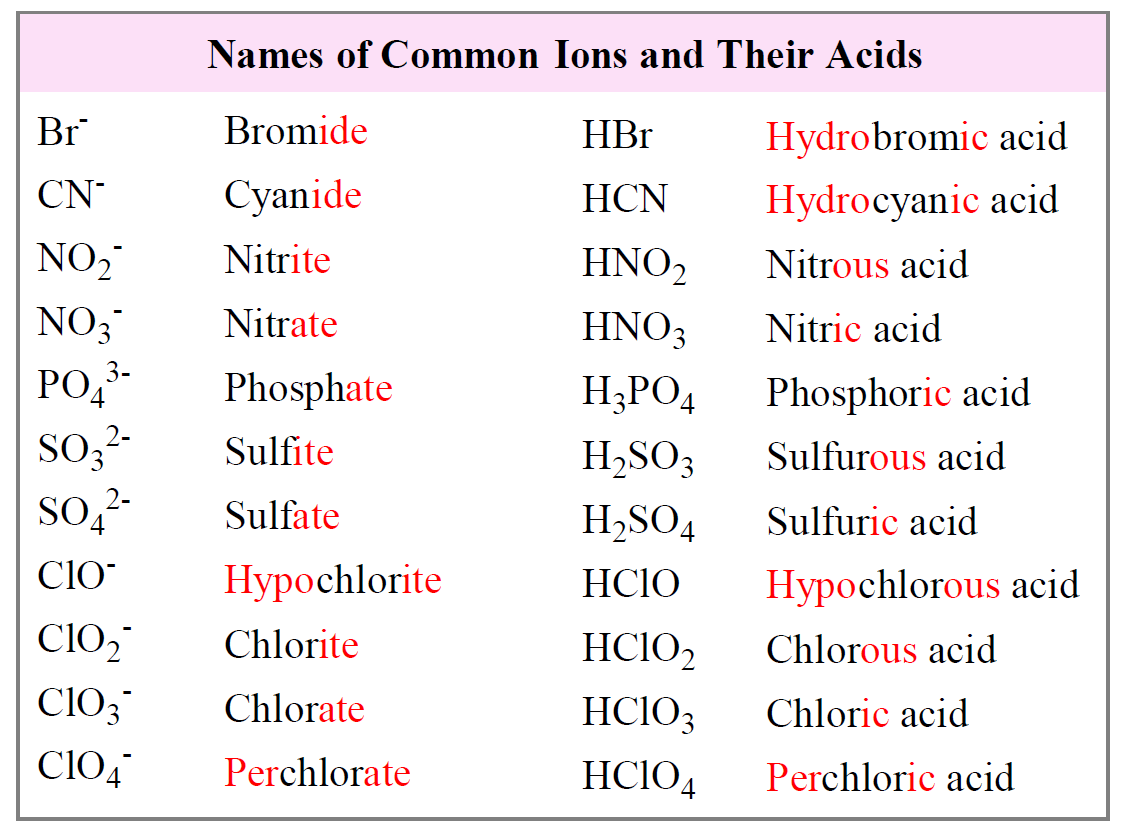
Oxyacids: Complex Yet Systematic
In contrast to binary acids, oxyacids involve polyatomic ions, such as sulfate (SO₄²⁻), nitrate (NO₃⁻), and phosphate (PO₄³⁻). Their nomenclature depends on the oxidation state of the central atom and the ion’s suffix. When these polyatomic ions are part of acids, the suffix “-ic” indicates a higher oxidation state (e.g., sulfuric acid from sulfate), while “-ous” denotes a lower oxidation state (e.g., sulfurous acid from sulfite). An oxyacid’s name derives from the parent ion’s name, adjusted with these suffixes, and often preceded by the word “hydro-” only if the acid is binary. For example, H₂SO₄ is sulfuric acid—indicating the high oxidation state—and H₂SO₃ is sulfurous acid.
| Relevant Category | Substantive Data |
|---|---|
| Suffix Rules | -ic for higher oxidation states, -ous for lower |
| Example: H₂SO₄ | Sulfuric acid |
| Example: H₂SO₃ | Sulfurous acid |
Comparison of Naming Rules: Confronting Challenges and Exceptions

The contrast between binary acids and oxyacids extends beyond their structural differences, touching upon their naming conventions’ complexities and exceptions. Binary acids’ straightforwardness facilitates quick learning but can obscure nuances, especially when dealing with less familiar nonmetals like iodine or bromine. Oxyacids demand a deeper understanding of polyatomic ions, oxidation states, and the historical conventions governing their suffixes.
Historical Development and Standardization of Acid Names
The systematic nomenclature we recognize today evolved from early alchemical traditions and gradually incorporated international standards, notably the International Union of Pure and Applied Chemistry (IUPAC). This standardization aimed to eliminate ambiguity and foster universal understanding. Over time, specific rules for oxyacid naming were formalized, accommodating common polyatomic ions and their oxidation states, leading to more consistent naming even in complex molecules. The binary acid naming conventions, although simpler, have retained their traditional format owing to their widespread use and simplicity.
| Relevant Category | Substantive Data |
|---|---|
| Standardization Timeline | Early 20th century reforms, adoption by IUPAC in the mid-1900s |
Practical Applications and Modern Usage in Chemical Industry
In contemporary practice, mastering acid naming rules directly impacts multiple sectors—from pharmaceuticals and environmental chemistry to materials science. Accurate naming influences documentation, patent claims, safety data sheets, and educational materials. For example, misnaming sulfuric acid in a safety protocol could lead to dangerous misunderstandings about concentration or handling procedures. The industrial context emphasizes the importance of both traditional and IUPAC-compliant nomenclature, which ensures clear communication amidst increasingly complex chemical formulations.
Advantages of Systematic Nomenclature in Industry
Adopting consistent naming conventions enhances traceability, reduces errors, and improves regulatory compliance. It also facilitates database searches and digital data management, where machine-readable nomenclature ensures interoperability. For instance, chemical inventories often utilize IUPAC names alongside CAS registry numbers, which serve as unique identifiers for acids and related compounds.
| Relevant Category | Substantive Data |
|---|---|
| Data Management | Improved searchability and interoperability using standardized names |
| Regulatory Compliance | Accurate documentation essential for safety and legal standards |
Common pitfalls and tips for mastering acid naming
Despite the clarity provided by established rules, learners often encounter pitfalls, such as confusing the suffixes “-ic” and “-ous” or misapplying the “hydro-” prefix. Additionally, exceptions—like the names of certain polyatomic ions—can challenge even seasoned chemists. To navigate these challenges, building a mental map of common ions and their oxidation states proves beneficial. Regular practice with example compounds, coupled with a deep understanding of their chemical context, can reinforce mastery.
Strategies to enhance learning and application
Utilize mnemonic devices to remember suffix rules; for example, “ic” relates to a higher state, and “ous” to a lower state. Employ visual aids, like flashcards with structures and names, to improve recall. Also, practicing naming and identifying acids from their formulas bridges theoretical knowledge with real-world application.
| Relevant Category | Practical Tips |
|---|---|
| Mnemonic Devices | "ic" = high, "ous" = low oxidation |
| Active Practice | Regular exercises in chemical nomenclature |
| Visual Aids | Structural diagrams and name association |
Future perspectives and ongoing developments in acid nomenclature

Looking forward, the evolution of chemical nomenclature continues alongside advances in analytical techniques and computational chemistry. Automated name generation via machine learning algorithms increasingly supports chemists, especially in high-throughput environments. As new acids—such as novel organofluorine compounds—emerge, nomenclature rules are occasionally revised for clarity and consistency. The integration of digital tools and cultural shifts toward open data further promote the standardization and dissemination of acid names across global scientific communities.
Emerging trends: Integration of automation and AI in nomenclature
Recent developments include AI-powered nomenclature systems capable of parsing complex molecular structures and generating correct IUPAC names instantaneously. This technology reduces human error and speeds up publication processes. Nonetheless, the foundational understanding of naming conventions remains critical to verify machine-generated names and ensure scientific accuracy.
| Relevant Category | Data and Trends |
|---|---|
| Automation Impact | Enhanced efficiency and reduced errors in nomenclature |
| Educational Shift | Increased emphasis on conceptual understanding alongside technological competence |
FAQs about Mastering Acid Naming Rules
What are the main differences between binary acids and oxyacids in naming conventions?
+Binary acids are named with the ‘hydro-’ prefix, the root of the nonmetal, and ‘-ic acid.’ Oxyacids are named based on the polyatomic ion with suffixes ‘-ic’ or ‘-ous’ indicating oxidation states, without the ‘hydro-’ prefix unless it’s a binary acid. The main difference lies in their structure and the suffix usage reflecting oxidation states.
Why is understanding oxidation states important in oxyacid naming?
+Oxidation states determine whether an oxyacid’s name ends with ‘-ic’ or ‘-ous.’ Accurately identifying these states ensures correct naming, which in turn affects clarity in chemical communication, especially in reactions involving different oxidation levels.
How do exceptions in acid naming rules affect learning?
+Some acids are named historically or irregularly, requiring learners to memorize exceptions. Recognizing patterns and understanding the underlying chemistry helps reduce confusion, but familiarity with common exceptions is essential for expert-level mastery.
What resources are most effective for mastering acid nomenclature?
+Textbooks, interactive online modules, practice exercises, and molecular modeling tools offer practical ways to reinforce rules. Engaging with real compounds and periodic table references streamlines the learning process.
